Tumor Associated Carbohydrate Antigens as Targets for Colorectal Cancer Immunotherapy
Summary
The present invention relates to a novel method to identify highly specific tumor associated carbohydrate antigens (TACAs) derived from colorectal cancer (CRC) tissue. Furthermore, it is believed that the markers disclosed may be useful therapeutic targets easily accessible as expressed on cell surfaces directly accessible to therapeutics and can be carried by multiple
proteins, reflecting the overall glycosylation phenotype of the cell, providing a broader tumor targeting strategy.
There was a patent filed recently which covers the workflow of extracting, identifying and analysing these highly specific structures as well as another one that includes the specific TACA structures which were found to be solely present in CRC and not in healthy colon providing a number of promising targets for potential therapy.
Background
Colorectal cancer (CRC) is one of the leading malignancies worldwide with over 900,000 deaths in 20201. Conventional therapeutic strategies include chemotherapy, radiation therapy, and surgery. However, due to poor screening strategies and lack of symptoms in early stages, most cases are detected at an advanced stage, leading to unsuccessful treatment. Unraveling the glycome in cancer is important for the development of immunotherapies for treating solid tumors and the discovery of cancer-specific glycan structures is critical for improving how these cancers are targeted.
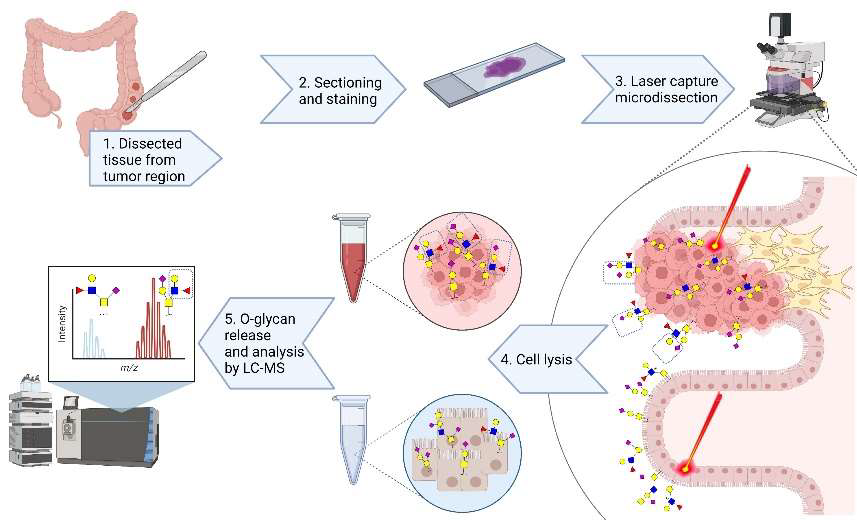
Technology
The present invention provides a better understanding of the variation in
O-glycosylation and its association with cell phenotypes. An in-depth
structural O-glycosylation analysis of 26 CRC cell lines was performed.
Additionally, the O-glycosylation signatures of primary cell lines as well as the primary and metastatic colorectal cancers they have been derived from were mapped and compared. Furthermore, glycosylation signatures of paired micro-dissected cancer and healthy mucosa were investigated. The released O-glycans were analysed on a sensitive nano-liquid chromatography coupled to a tandem mass spectrometer using electrospray ionization enabling powerful separation of isomeric species, as well as in-depth structural characterization of the epitopes. The major outcomes were as follows:
- Using transformative technology based on laser capture microdissection allowed for the first time to dissect the cancer cell O-glycome of CRC.
- Highly specific glycan structures were observed in cancer cells from most of the tumor tissue which were not present in healthy control tissue.
Value Proposition
The global colorectal cancer therapeutics market is poised to grow by USD 994.94 million during 2019-2023, progressing at a CAGR of almost 3% (www.businesswire.com).
Team
Prof Manfred Wuhrer currently holds the position as Head of the Center for Proteomics and Metabolomics (CPM) at the Leiden University Medical
Center in Leiden/ The Netherlands. Dr Guinivere Lageveen Kammeijer is
Senior Scientist at the CPM focusing on exploring cancer glycosylation for developing novel therapeutic and diagnostic applications. Dr Katarina
Madunic is Scientist at the CPM specialized in dissecting cancer cell
glycosylation.
Key benefits
- The LC-MS approach allowed for untargeted discovery of cancer antigens from tissues.
- A specific set of TACAs relating to CRC was identified which could provide a promising set of targets for therapy.
Applications
- Identification of cancer targets with high confidence. Lack of presence in healthy controls.
- Invention could be used for the further development of cancer
- specific therapeutics targeting the identified TACAs.
- TACAs could be identified for other cancer types.
Luris reference number
INV-21MC00834Patent status
This technology is subject of two Dutch patent applications.



