Targeting ligand-bound (solid-phase) C1q
SUMMARY
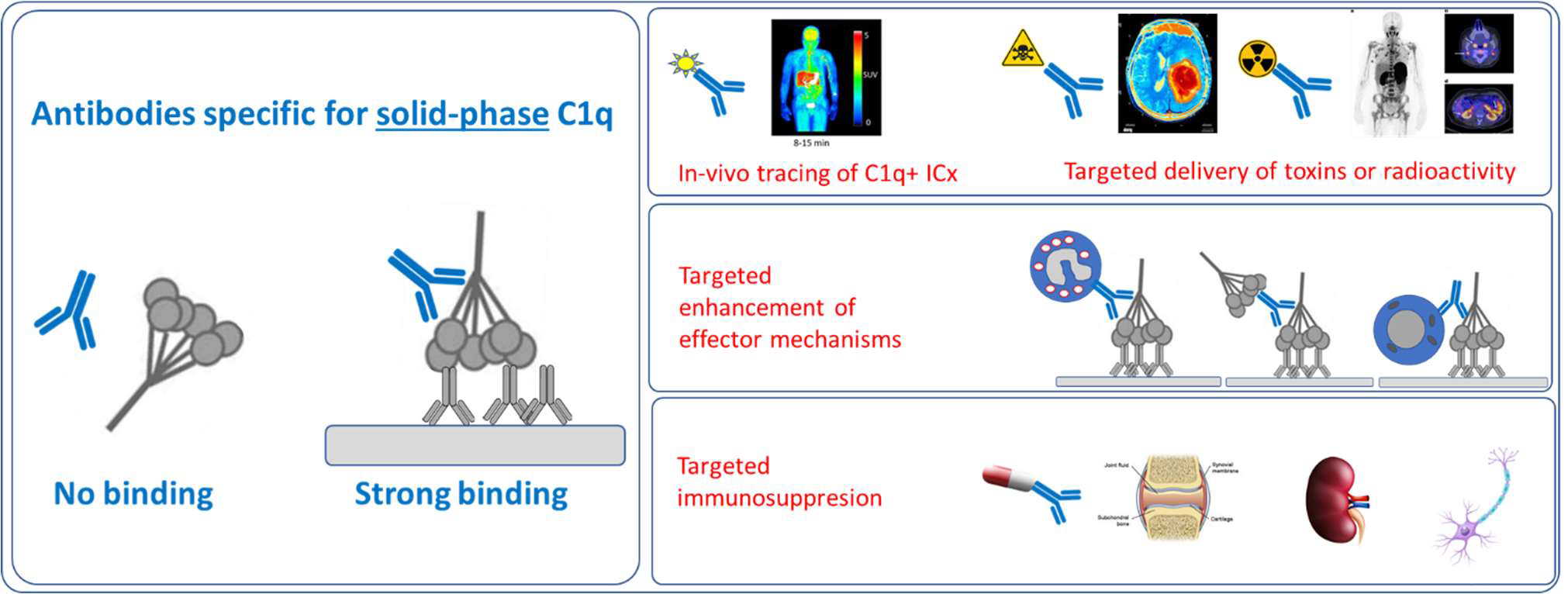
We have recently developed recombinant fully human antibodies that strongly bind to C1q that is bound to its ligands (solid-phase) but does not bind to circulating C1q. This property provides unique opportunities as now these antibodies can be used to target C1q in immune complexes or C1q in specific tissues without interference of circulating C1q. Binding to circulating C1q would not only cause a huge sink for any
therapeutics or tracers, but it would also interfere with the normal C1q functions, cause clearance of C1q and reduced complement activity. Using t
BACKGROUND
C1q is the recognition molecule of the classical pathway of complement activation. C1q circulates in the blood at a concentration of around 150 μg/ml. C1q only acquires the capacity to activate the complement system after binding to an array of its ligands. These ligands include for example, immune complexes comprising antigen bound IgG antibodies or antigen bound IgM as well as ligand bound C-reactive protein. If these ligands are present in a sufficiently multimeric format than C1q binds, which results in activation of the C1 enzymes, C1r and C1s and subsequent complement activation takes place. These processes are beneficial in fighting infections and in fighting tumors, but unfortunately do also occur on host tissues where e.g. autoantibodies accumulate. Blocking C1q and classical pathway activity by antibodies has been performed, it depletes all circulating C1q and blocks classical pathway activity, however this works systemically and may put the patient in danger of infections and development of autoimmunity. Here we describe the use of very different anti-C1q autoantibodies, now targeting only ligand-bound C1q while not binding to / interfering with circulating C1q. Such anti-C1q autoantibodies have been described, also by our team, to occur in lupus patients and in some controls. They were shown to be pathogenic, but only if ligand-bound C1q was present in target organs. Obviously, the developed antibodies will be engineered to acquire the desired immune effector properties. Here we describe new, completely human antibodies that we produce recombinantly. This allows us to modify the Fc-domain of the antibody in such a way that it can no longer bind Fc-Receptors or trigger additional complement activity. In this format the antibodies can be safely used as tracers or as antibody drug conjugates. In conditions where one would like to amplify inflammation we can introduce mutations that will enhance Fc-Receptor interactions, that will boost complement activity or that may engage specific immune cells to fight cancer or (antibiotic resistant) infections.
TECHNOLOGY
The invention builds on the use of antibodies that bind to solid-phase C1q. Combined with available technologies we can generate antibodies with inert or active Fc-domains regarding immune activation. In addition we can generate tracers, and antibody-drug conjugates.
VALUE PROPOSITION
The global complement-targeted therapeutics market is expected to
grow at a CAGR of 10.5% from 2022 to 2030. The growth in this
market can be attributed to the increasing prevalence of complement-mediated diseases, rising demand for novel therapies, and growing investments in R&D by pharmaceutical companies.
TEAM
Dr. Leendert Trouw currently holds a position as Professor at the
Department of Immunology at the Leiden University Medical Center
in Leiden, The Netherlands.
Key benefits
- These anti-C1q antibodies
- Only bind C1q that is ligand-bound (solid-phase)
- No antibody sink from circulating C1q
- No C1q depletion or block of CH50
- Specific targeting to locations where C1q is accumulating in ligand-bound format
- Enhancing effector functions to tumors and resistant infections
Applications
- Applicable to a wide range of autoimmune diseases, transplantation, cancer and (resistant) infections
- The current invention may also be used for imaging, monitoring, diagnosis, prognosis, personalized medicine and therapy
- Can be used to inform clinicians when to start but also when to stop
- (expensive) complement inhibitory therapy or other immunosuppression
- Relevant as a noninvasive tool to monitor organ involvement and flares in Lupus
Luris reference number
??Patent status
This technology is subject of a patent application (Dutch Patent Application No 2033789)



